For the first time, scientists have mapped the distribution of electrons in the outermost shell – the so-called valence electrons – of atoms in an organic molecule.
The experimental breakthrough sheds light on the fundamental nature of chemical bonds. It could have implications for pharmaceuticals and chemical engineering.
The behavior of electrons in atoms depends on the orbitals they occupy in the atomic shell. These orbitals can be roughly imagined as how close the electron orbits the atomic nucleus.
Different conventions name these shells differently (for example, starting with the innermost shell: 1,2,3, …; s, p, d, …; or K, L, M, …).
Electrons closer to the nucleus, so-called inner shell or core electrons, provide stabilization and do not interact with other atoms.
However, outer shell electrons describe the properties of the material, including how the atoms form chemical bonds with other atoms. The number of valence electrons depends on the element’s position in the periodic table and certain rules regarding the number of electrons that fit in each orbital shell.
As you go through the periodic table, you’ll see that the number of electrons in an atom increases. For example, carbon is the 6th element in the periodic table and, when not ionically charged, has 6 electrons. Two of these go into the first shell and the other 4 go into the second shell, which can hold a maximum of 8 electrons before the third shell is filled. Their 4 valence electrons make carbon atoms very receptive to chemical bonding.
However, obtaining information about valence electrons is challenging. Until now, researchers had to rely on theoretical models and spectroscopy to estimate the behavior of valence electrons.
In the new study, published in Journal of the American Chemical SocietyThe researchers used synchrotron X-ray diffraction experiments to image only the valence electrons.
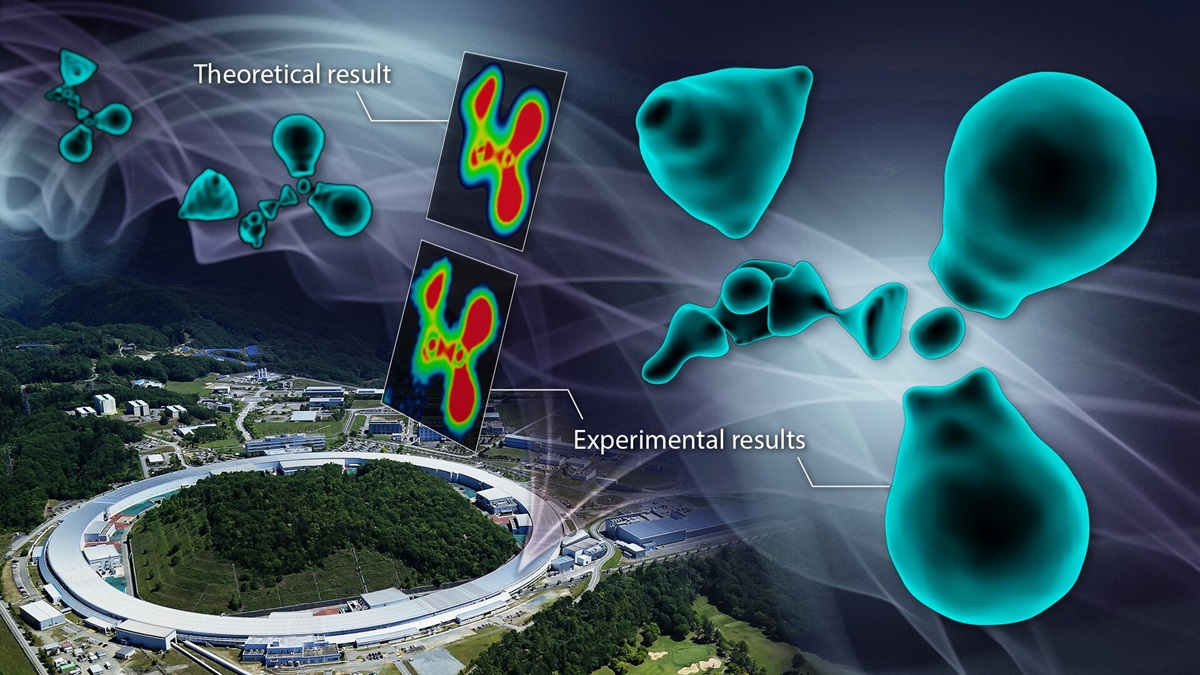
The experiments were carried out at the Super Photon Ring 8 GeV facility in Japan.
“Using this method, we observed the electronic state of the glycine molecule, a type of amino acid,” says corresponding author Hiroshi Sawa of Nagoya University. “Although the method was relatively simple to perform, the result was impressive.”
Sawa says that contrary to expectations, the valence electrons appeared as individual clumps.
“The observed electron cloud did not have the smooth, enveloping shape that many had predicted, but rather a fragmented, discrete state.”
By translating the image into a color map, the researchers discovered that there are interruptions in the electron distribution around the carbon atoms in the molecule.
“When carbon forms bonds with surrounding atoms, it reconstructs its electron cloud to create hybridized orbitals,” explains Sawa.
Because of this hybridization and the wave-like nature of the electrons, instead of a continuous “cloud” of electrons, there are parts of the hybrid orbitals where no electrons are present.
This surprising result was confirmed by quantum chemical calculations performed by researchers at Hokkaido University.
“I think it helped to draw a clear conclusion to the ambiguous understanding of bonding states that has puzzled researchers since the 19th century,” says Sawa. “Visualizing the behavior of electrons is a challenging endeavor.”
“This study has made it possible to directly visualize the nature of chemical bonds, which may contribute to the design of functional materials and the understanding of reaction mechanisms,” Sawa adds. “Because it helps in discussing the electronic states of molecules, which are difficult to deduce from the chemical structure formula.”
“For example, it could explain why some drugs work and others don’t. Areas where interactions affect functionality and structural stability, such as organic semiconductors and the study of the structure of DNA double helixes, are likely to benefit most from our research.
“I think our results surprised many researchers and confirmed the model proposed by quantum chemistry.”

:max_bytes(150000):strip_icc():focal(626x277:628x279)/Space-X-Polaris-Dawn-053024-107d69ad507c4c7c99015c60b9747f4f.jpg)

