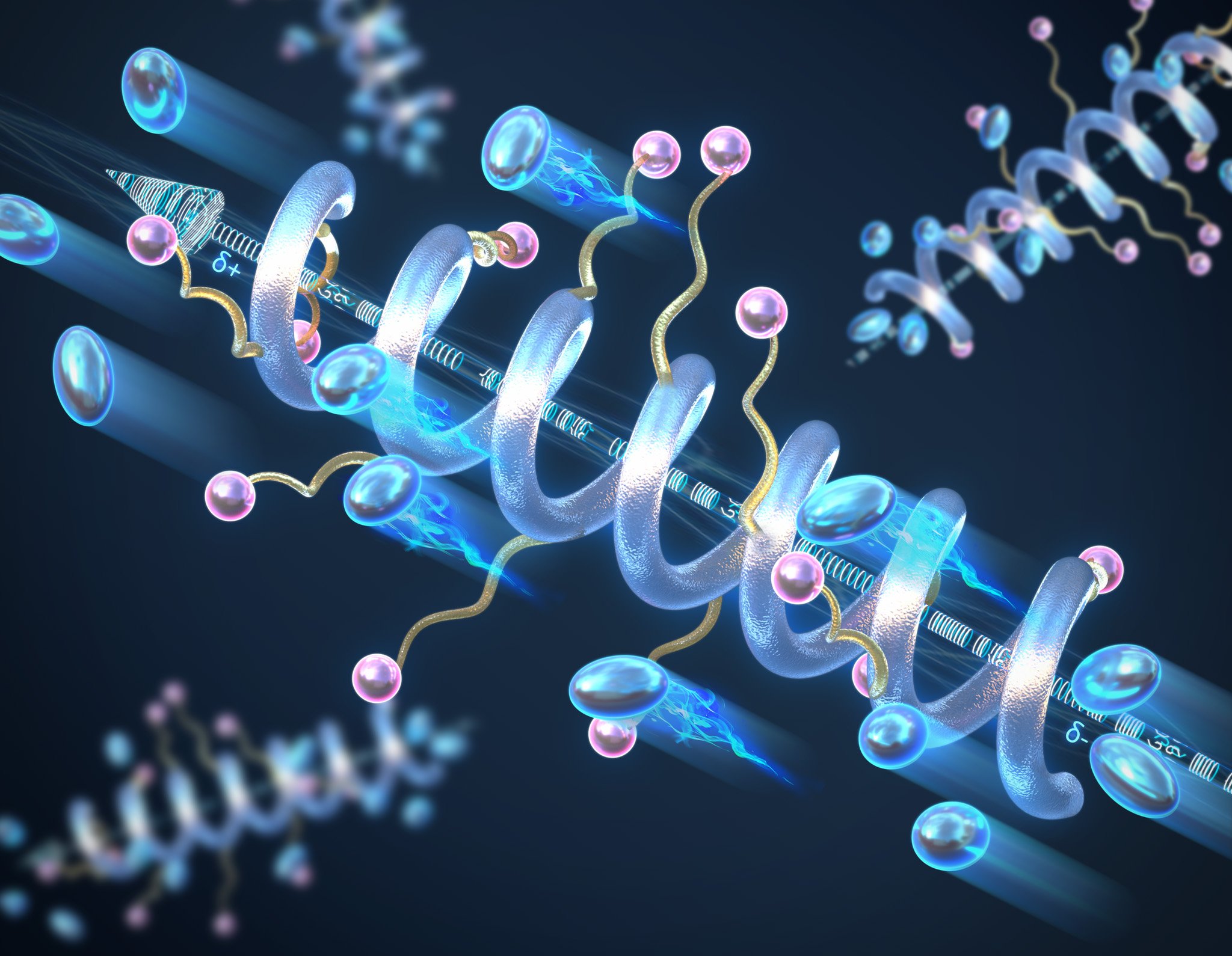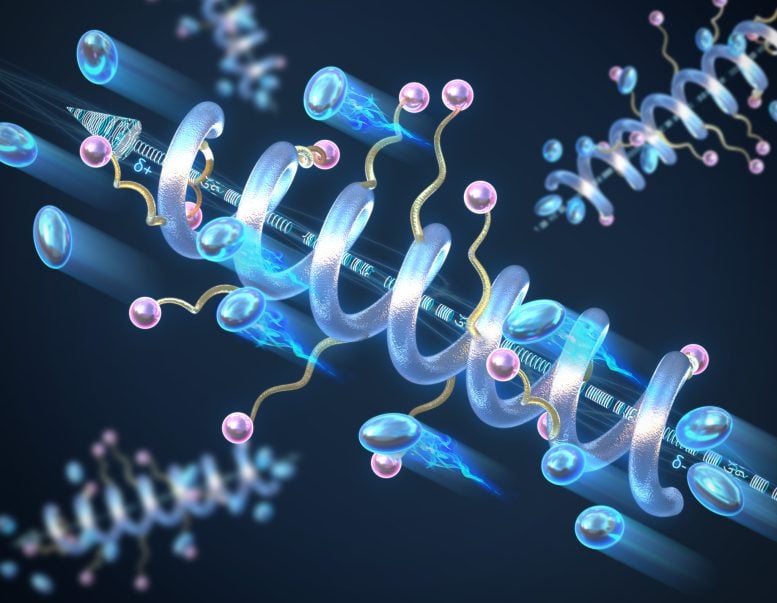
Researchers at the University of Illinois Urbana-Champaign have developed spiral-structured peptide polymer electrolytes that exhibit better conductivity and stability compared to conventional random coil structures. These spiral-shaped polymers improve the performance of solid-state batteries and are environmentally friendly because they can be decomposed and recycled after their useful life.
Researchers have been studying solid-state electrolytes for decades as possible components in energy storage systems, particularly for the development of solid-state batteries. These materials are safer alternatives to the traditional liquid electrolytes – a solution that allows ions to move within the cell – used in batteries today. However, new concepts are needed to boost the performance of current solid polymer electrolytes to make them viable for next-generation materials.
Materials scientists and engineers at the University of Illinois Urbana-Champaign have investigated the role of helical secondary structure on the conductivity of solid-state peptide polymer electrolytes and found that the helical structure exhibits significantly higher conductivity compared to its random coil counterparts. They also found that longer helices lead to higher conductivity and that the helical structure increases the overall stability of the material to temperature and voltage.
“We introduced the concept of using a secondary structure – the helix – to develop and improve the fundamental material property of ionic conductivity in solid materials,” says Professor Chris Evans, who led this work. “It’s the same helix you would find in peptides in biology, we’re just using it for non-biological reasons.”
Improvements through helical structures
Polymers tend to adopt random configurations, but the backbone of the polymer can be controlled and designed to form a helical structure, such as DNA. As a result, the polymer will have a macrodipole moment – a large-scale separation of positive and negative charges. Along the length of the helix, the small dipole moments of each individual peptide unit will add up to form the macrodipole, which increases both the conductivity and the dielectric constant – a measure of a material’s ability to store electrical energy – of the entire structure and improves charge transport. The longer the peptide, the higher the conductivity of the helix.
Evans adds: “These polymers are much more stable than typical polymers – the helix is a very robust structure. Compared to random coil polymers, they can be subjected to high temperatures or stresses without the helix being degraded or lost. We see no signs of the polymer breaking down sooner than we would like.”
Since the material consists of peptides, it can also be broken down by enzymes or acid when the battery is defective or has reached the end of its useful life. The raw materials can be recovered and reused after a separation process, which reduces the environmental impact.
Reference: “Helical peptide structure improves conductivity and stability of solid electrolytes” by Yingying Chen, Tianrui Xue, Chen Chen, Seongon Jang, Paul V. Braun, Jianjun Cheng and Christopher M. Evans, August 6, 2024, Natural materials.
DOI: 10.1038/s41563-024-01966-1
This research was funded by the U.S. National Science Foundation and the U.S. Department of Energy (Office of Basic Science, Division of Materials Science and Engineering).